CAMBRIDGE, Mass., 01/11/2023 – ConcertAI’s TeraRecon, a leader in advanced visualization and AI Clinical SaaS, is pleased to announce an expanded collaboration with Avicenna.AI, a specialist in medical imaging AI. Together, they will provide global solutions that leverage artificial intelligence to enhance care coordination and improve patient triage for individuals afflicted with pulmonary embolism (PE) and aortic dissection (AD). This collaboration aims to expedite clinical decision-making and optimize patient care for these critical medical conditions.
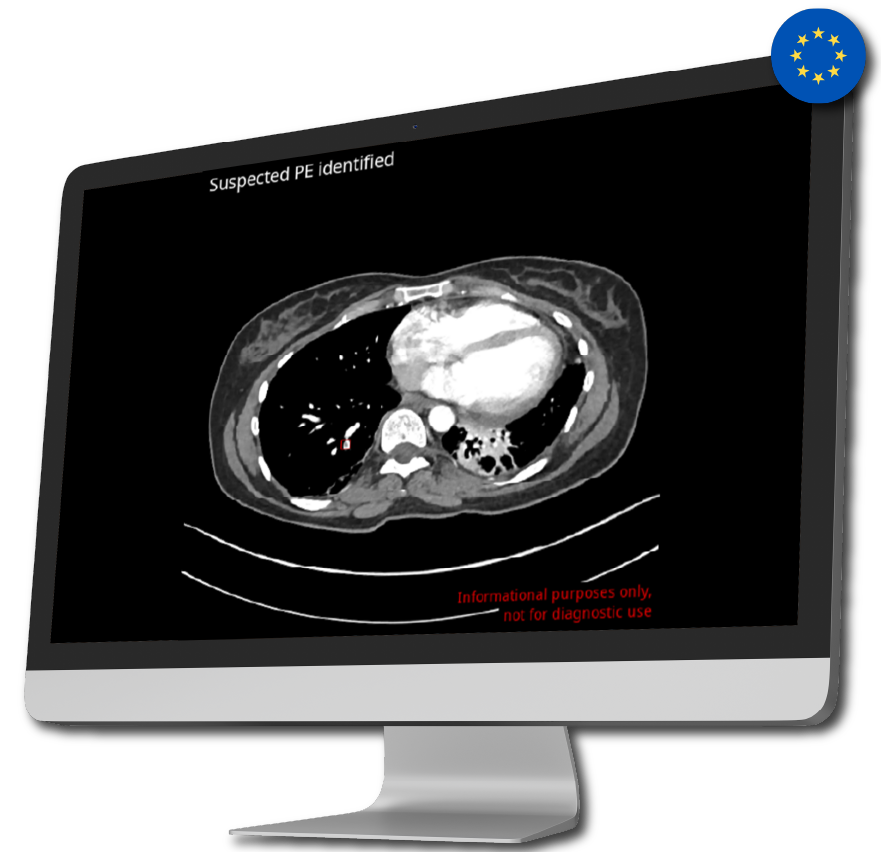
Diagnosing and coordinating care for patients facing PE and AD pose significant hurdles. Avicenna.AI has FDA approval for its algorithms, tailored for PE and both Type A and Type B AD, which will seamlessly integrate into the Eureka Clinical AI platform. This integration aims to streamline identification of these conditions, thus empowering multidisciplinary patient care coordination. Upon detecting a PE or AD, the Eureka Clinical AI platform will rapidly notify each provider’s desktop or mobile device, delivering dynamic imaging and comprehensive patient information. Additionally, the aortic module offering will grant access to imaging and workflows supporting coordinated care for patients with abdominal aortic aneurysm (AAA), thoracic aortic aneurysm (TAA), rupture, stenosis, and transection, alongside the AD algorithm. Likewise, the PE algorithm will be accessible through the Eureka Clinical AI platform’s PE module.
The inclusion of AI-powered workflows via the Eureka Clinical AI platform is anticipated to significantly reduce the time it takes a physician to diagnose these potentially life threating conditions to treatment by facilitating more informed treatment decisions across health systems.
Through the incorporation of Avicenna’s PE and AD solutions, coupled with the existing CINA-iPE on the Eureka Clinical AI platform for the EU, TeraRecon is positioned to offer enhanced pulmonary and aortic solutions to its global clientele of approximately 1,900 customers.
“We are dedicated to expanding the Eureka Clinical AI ecosystem with impactful AI solutions that support our clinicians and care providers,” said Dan McSweeney, President of TeraRecon. “Avicenna’s solutions play a crucial role in fulfilling this commitment by broadening access to essential triage capabilities through our scalable Eureka Clinical AI platform.”
Avicenna.AI specializes in providing healthcare AI solutions that utilize deep learning to identify, detect, and quantify life-threatening pathologies from CT images. By employing a blend of deep learning and machine learning technologies, the company’s solutions autonomously detect and prioritize emergency cases within seconds, assess their severity, and promptly notify radiologists.
“We are excited to empower TeraRecon users worldwide with our groundbreaking AI-driven solutions for the care coordination of pulmonary embolism and aortic dissection,” said Cyril Di Grandi, Chief Executive Officer of Avicenna.AI. “Our aim is to expedite an optimal medical response within a short timeframe and enhance patient care. We eagerly anticipate extending the benefits of our pulmonary embolism and aortic dissection triage tools to the emergency room and beyond.”
Eureka Clinical AI stands as the foremost AI SaaS imaging interpretation and clinical decision augmentation solution from ConcertAI’s TeraRecon. Distinguished as the industry’s most widely deployed platform, it uniquely accommodates third-party AI algorithms, facilitating consolidated management of all AI interpretation solutions with seamless PACS integrations. Multi-specialty care teams can swiftly access results and receive mobile alerts to confirm AI findings, ensuring optimal and timely patient interventions, management, and coordinated care delivery.
To learn more about the Eureka Clinical AI platform, its capabilities, and algorithms spanning neurology, radiology, cardiology, oncology, and more, visit: https://www.terarecon.com/artificial-intelligence.
About TeraRecon: Serving approximately 1,900 clinical sites globally, TeraRecon, a ConcertAI company, is a Best in KLAS solution provider for AI-empowered radiology, oncology, cardiology, neurology, and vascular surgery. Awarded the KLAS Category Leader for Advanced Visualization, TeraRecon solutions are independent of any one manufacturer’s imaging equipment or PACS, allowing a single, unified, and simplified clinical workflow that can improve efficiencies and deliver actionable physician-guided insights. For more information, visit us at www.terarecon.com
About ConcertAI: ConcertAI is the leader in Real-World Evidence (RWE) and AI technology solutions for life sciences and health care. Our mission is to accelerate insights and outcomes for patients through leading real-world data, AI technologies, and scientific expertise in partnership with the leading biomedical innovators, health care providers, and medical societies. For more information, visit us at http://www.concertai.com or follow us on LinkedIn.
About Avicenna.AI: Founded in 2018, Avicenna.AI develops medical imaging AI solutions for highly prevalent pathologies. The company uses artificial intelligence and deep learning to optimize many of a radiologist’s manual tasks. Its CINA products leverage deep learning algorithms to identify acute abnormalities and to enhance clinicians’ workflow. Avicenna.AI is co-founded by Cyril Di Grandi, who previously co-founded and successfully sold Olea Medical, and Dr. Peter Chang, a radiologist and internationally recognized expert in AI and deep learning. www.avicenna.ai