In trauma care, every second counts. When a patient arrives in the emergency department with a suspected intracranial hemorrhage (ICH) or cervical spine fracture, the clock is ticking. A delayed diagnosis can mean the difference between recovery and irreversible neurological damage, permanent disability, or even death.
Despite the urgency, these life-threatening conditions can be subtle to detect. Overburdened radiologists must analyze hundreds of scans in a high-pressure environment, where even the smallest delay can have devastating consequences. This is where AI is transforming emergency medicine.
The Race Against Time in Emergency Trauma Cases
Intracranial Hemorrhage: A Silent Killer
Intracranial Hemorrhage occurs when bleeding inside the skull puts dangerous pressure on the brain. It can result from head trauma, stroke, or a brain aneurysm rupture, and its symptoms—confusion, headaches, loss of consciousness—are often subtle, making immediate detection critical.
Why speed matters?
- Delayed detection can lead to irreversible damage
- Increased intracranial pressure can cause brain herniation
- Patients risk permanent neurological deficits or even death
Standard diagnosis relies on radiologists reviewing non-contrast CT scans. But in a busy ER, radiologists may be overwhelmed, leading to delays or potential misdiagnoses.
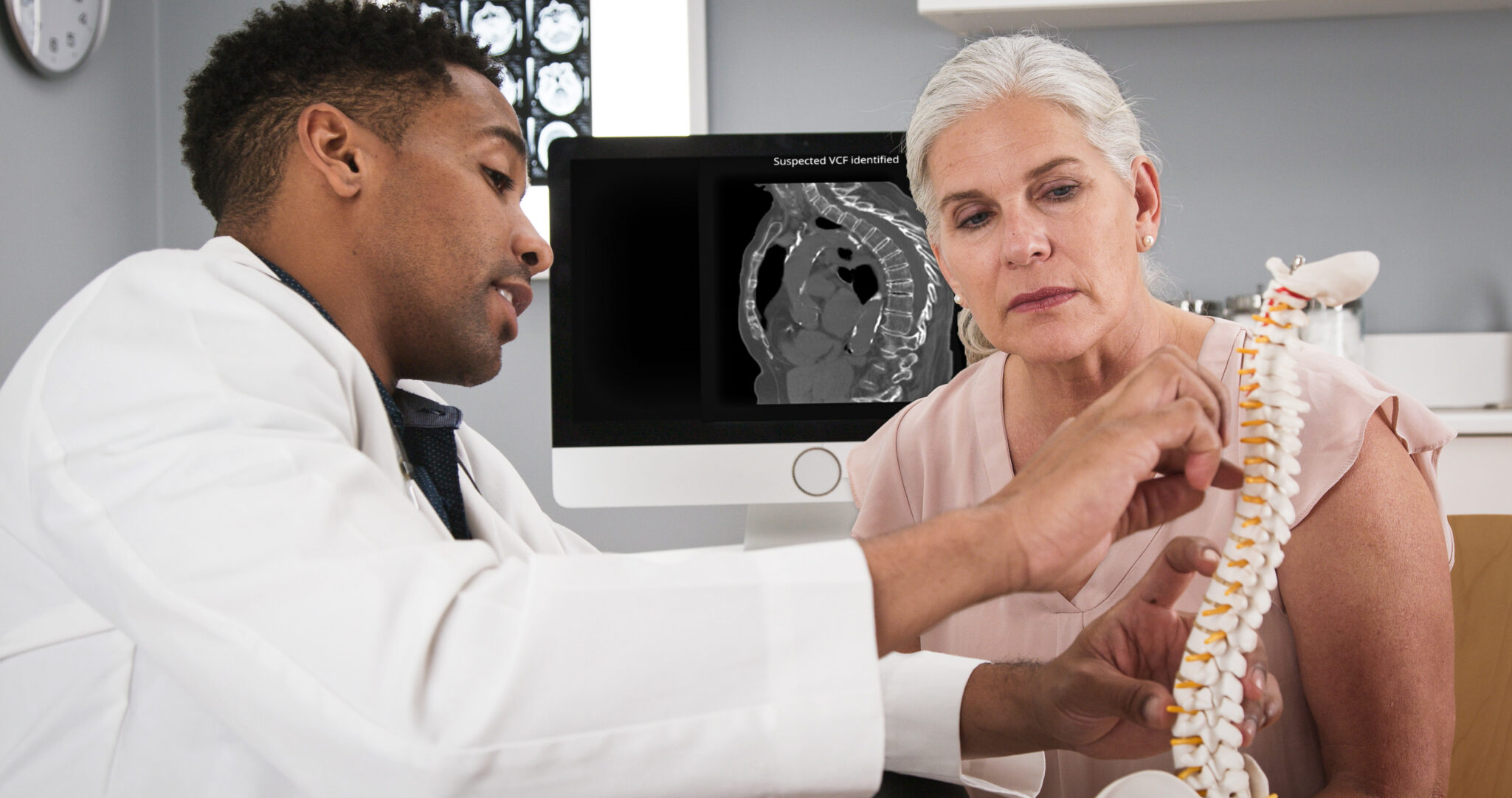
Cervical Spine Fractures: A Hidden Threat
Cervical spine fractures occur when one of the seven neck vertebrae breaks, often due to car accidents, falls, or sports injuries. These fractures require urgent attention and can be easily overlooked by radiologists, yet failing to diagnose them in time can lead to devastating consequences.
The risks of delayed cervical spine fractures:
- Unstable fractures can worsen with movement
- Spinal cord injury may result in paralysis
- High cervical spine injuries (C1-C4) can cause respiratory failure
Like ICH, detecting cervical spine fractures requires careful CT scan interpretation—but subtle fractures can be overlooked in fast-paced emergency settings.
How CINA Trauma is Transforming Trauma Diagnosis?
CINA Trauma is changing the game, providing faster, more accurate assessments of intracranial hemorrhage and cervical spine fractures, ultimately improving patient outcomes.
Faster Prioritization, Reduced Delays
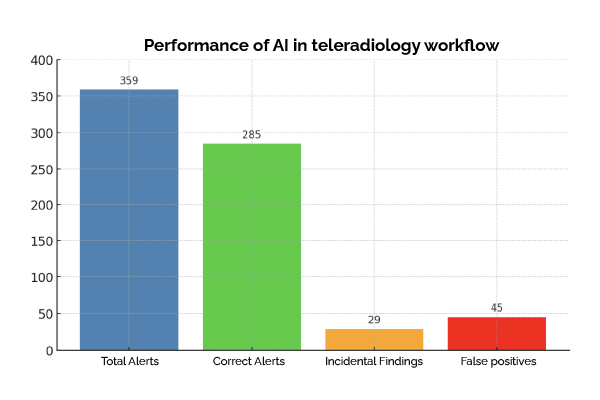
CINA Trauma analyzes non-contrast CT scans in seconds, automatically detecting ICH and cervical spine fractures. Positive cases are flagged for priority review, ensuring that critical patients receive immediate care.
Supporting Overburdened Radiologists
More experienced radiologists are frequently interrupted to assess scans, even when most results are negative. AI is a safety net, reducing unnecessary disruptions while ensuring no critical case goes unnoticed.
Enhancing Accuracy, Reducing Missed Diagnoses
Both ICH and cervical spine fractures can be subtle, especially in polytrauma cases where multiple injuries require assessment. AI acts as an extra layer of precision and security, helping radiologists catch what the human eye might miss.
Optimizing Emergency Workflow
AI-powered detection streamlines trauma care by:
✅ Instant AI-Powered Triage: Automatically prioritizes critical cases, ensuring timely intervention and preventing delays in emergency care.
✅ Reduced Burden on On-Call Radiologists: Minimizes unnecessary overnight disruptions by filtering out false alarms, allowing radiologists to focus on urgent cases.
✅ Enhanced Patient Outcomes: Enables earlier surgical intervention and improves patient outcomes
✅ Mitigated Legal Risks for Hospitals & Physicians: Acts as a safety net, reducing the likelihood of missed diagnoses and enhancing diagnostic confidence.
🚀 Want to try CINA Trauma on your clinical routine? Request a meeting with one of our experts.







 “We are delighted to launch CINA-ASPECTS to the US market, marking a significant milestone as our first CADx product receives FDA clearance,” said
“We are delighted to launch CINA-ASPECTS to the US market, marking a significant milestone as our first CADx product receives FDA clearance,” said 