“Time is brain” is a well-known adage, particularly for patients with head trauma and stroke, where every minute counts. Avicenna.AI provides efficient solutions not only for the detection of traumatic brain injury (TBI) and hemorrhagic stroke, but also for the identification and quantification of acute ischemic stroke. Our triage tools enable fast detection to improve clinical workflow. We address data overload and fatigue to reduce errors and misdiagnosis.
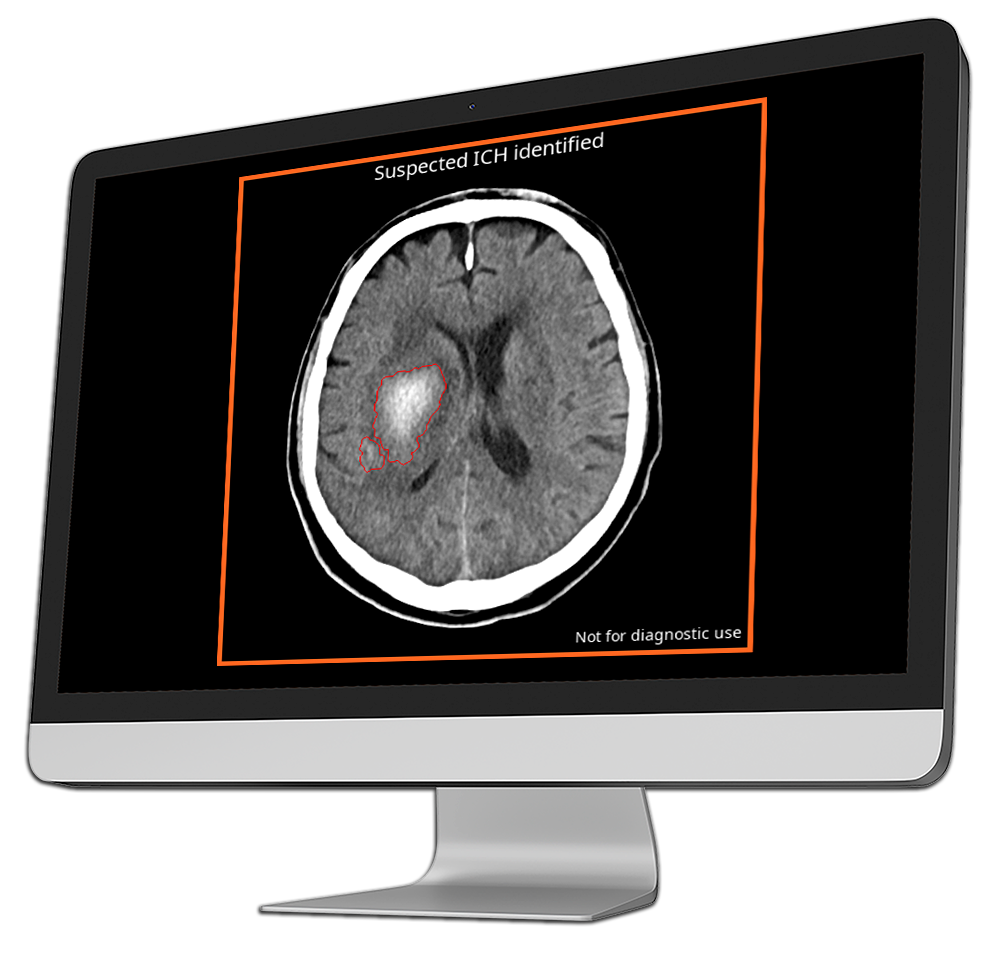
CINA-ICH is a triage tool that detects intracranial hemorrhages on non-enhanced head CT scans, identifying bleeding caused by hemorrhagic strokes and traumatic brain injuries.
By rapidly flagging these life-threatening conditions, CINA-ICH enables radiologists to prioritize critical cases, significantly reducing diagnosis time and allowing faster intervention, which can be crucial in preventing long-term brain damage and improving patient outcomes.
CINA-ICH detects all types of intracranial hemorrhages, including intraparenchymal, intraventricular, subarachnoid, subdural, and epidural bleeds.
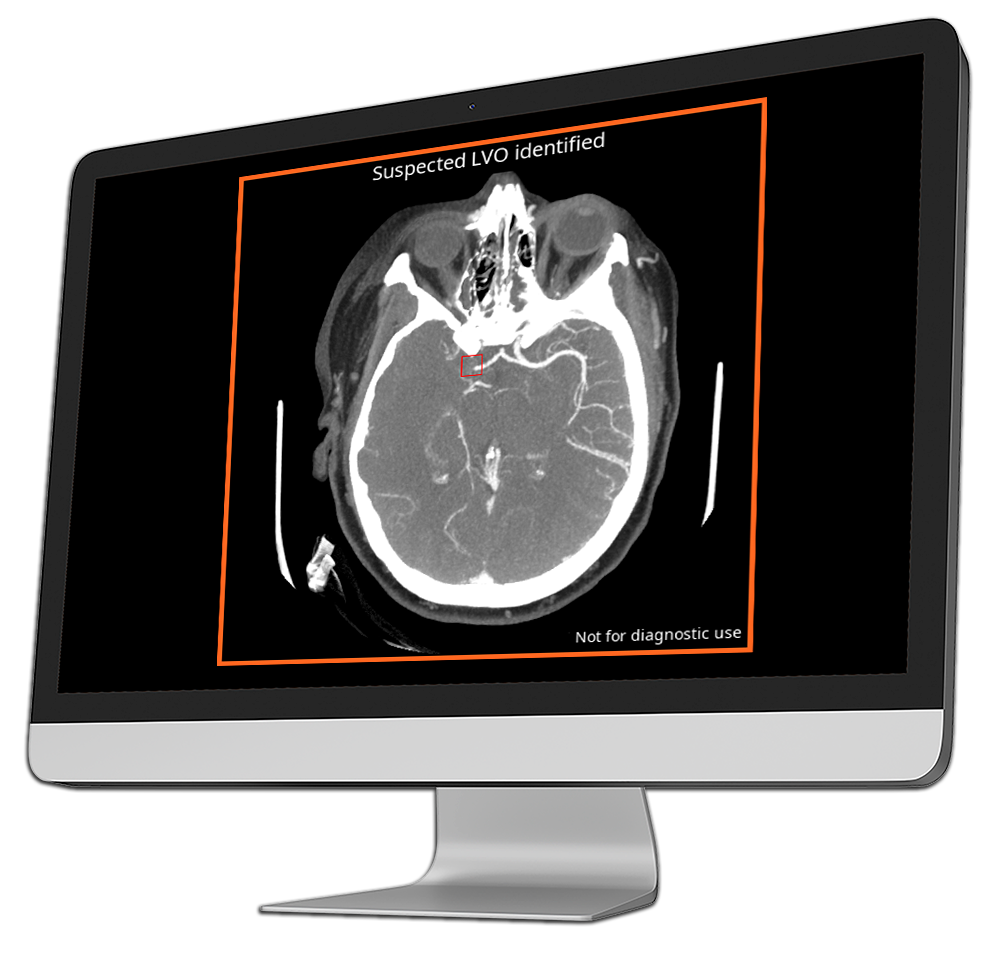
Nearly 80% of ischemic strokes are caused by large vessel occlusions (LVOs) – every 10 minutes of delay can mean a lifetime of disability.
CINA-LVO is a triage tool that detects large vessel occlusions on CT angiography scans, identifying blockages in major intracranial arteries that are commonly caused by ischemic strokes.
CINA-LVO detects occlusions in key anterior circulation vessels, including the internal carotid artery (ICA) and the middle cerebral artery (MCA, M1 and M2 segments).
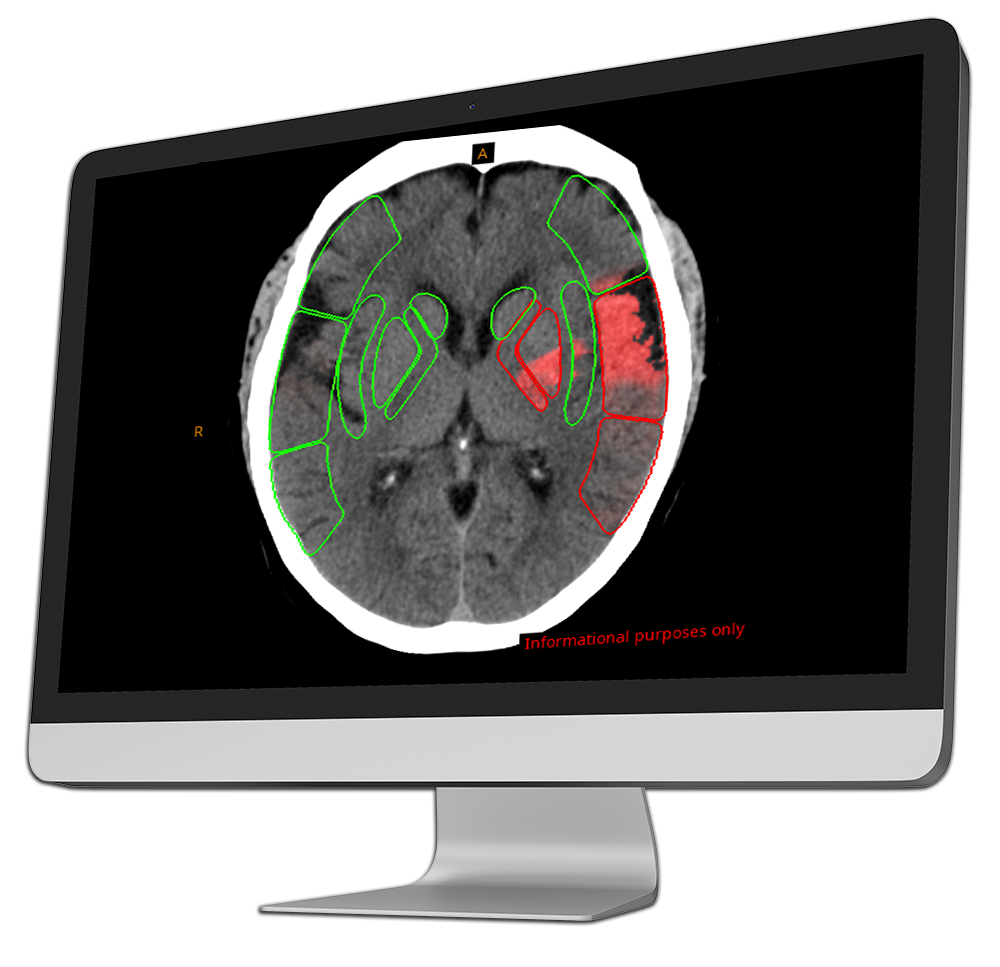
CINA-ASPECTS analyzes non-enhanced head CT scans to calculate the Alberta Stroke Program Early CT Score (ASPECTS), helping identify early ischemic changes in acute stroke patients.
By delivering a rapid and objective score, it supports radiologists in assessing stroke severity, ensuring consistency, and accelerating treatment decisions.
In addition to the ASPECTS score, CINA-ASPECTS provides a heat map showing the probability of hypodensity or sulcal effacement and outlines infarcted regions, enhancing evaluation of patients eligible for mechanical thrombectomy or intravenous thrombolysis.
CINA and CINA-ASPECTS, medical images analysis softwares, are medical devices manufactured by Avicenna.AI. These medical devices are reserved for health professionals. The performance and outputs of these tools may differ slightly according to the software version and applicable regulations.
These software have been designed and manufactured according to the EN ISO 13485 Quality management system. Read the instructions in the notice carefully before any use.
Complete the form below to download our Case Study.