Avicenna.AI lands FDA clearance for AI tool for cervical spine fracture detection
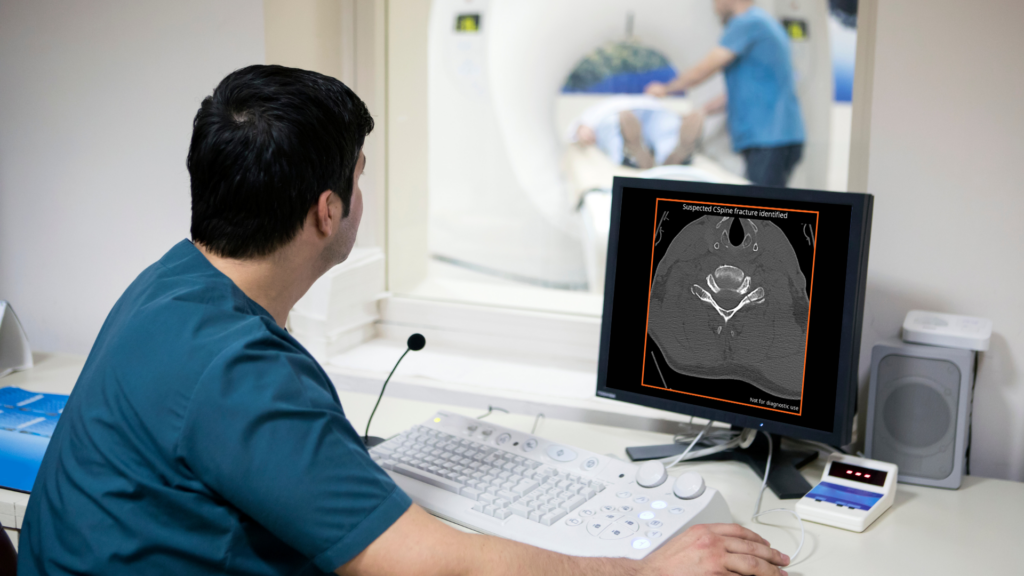
Medical imaging AI company receives approval for CINA-CSpine algorithm enabling detection and triage of cervical spine fractures. La Ciotat, FRANCE – September 19, 2024 – Medical imaging AI company Avicenna.AI today announced that it has received 510(k) clearance from the US Food and Drug Administration for its CINA-CSpine tool. Using a combination of deep learning […]
Avicenna.AI secures Medical Device Regulation certification for medical imaging AI portfolio

French AI company achieves key EU regulatory compliance for multiple algorithms that identify, detect, and quantify severe conditions from medical images.
Avicenna.AI secures FDA clearance for AI detection of vertebral compression fractures
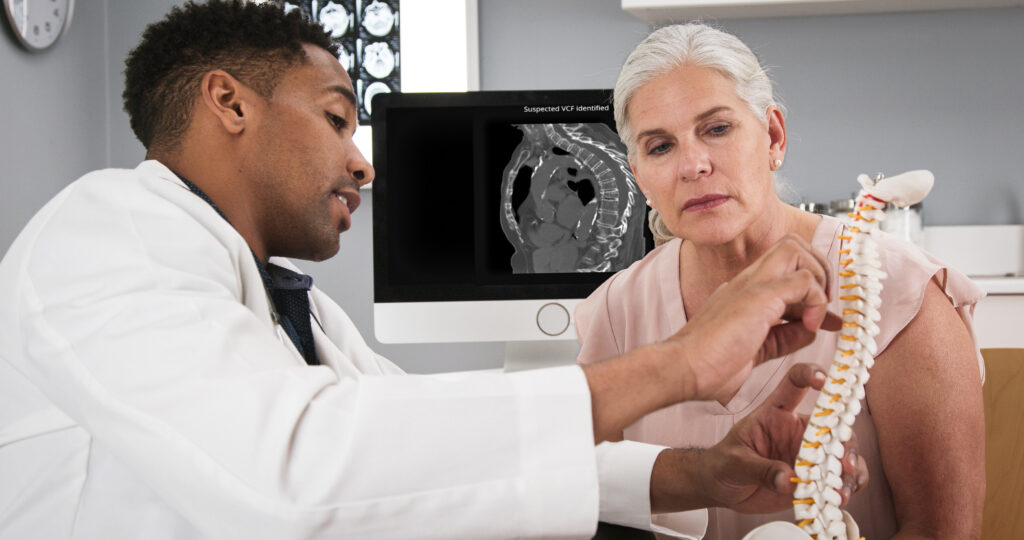
Avicenna.AI receives approval for algorithms enabling incidental detection of pulmonary embolism and automatic assessment of stroke severity.
Avicenna.AI signs a distribution agreement with Sectra for neurovascular AI solutions

Avicenna.AI signs a distribution agreement with Sectra for neurovascular AI solutions
Avicenna.AI partners UpCare for innovative value-based solutions to Canadian health providers

Avicenna.AI Partners With UpCare To Deliver Innovative Value-Based Solution to Canadian Health Providers
Viz.ai and Avicenna.AI Launch AI Care for Pulmonary Embolism & Aortic Disease

Viz.ai and Avicenna.AI Partner to Launch World-class AI-Driven Intelligent Care Coordination for Pulmonary Embolism and Aortic Disease
Avicenna.AI Joins Nuance AI Marketplace, Offering Cutting-Edge Solutions for Diagnostic Imaging

Avicenna.AI Joins Nuance AI Marketplace for Diagnostic Imaging for Stroke tools
AI triage solution for deadly vascular conditions receives FDA clearance and CE Mark
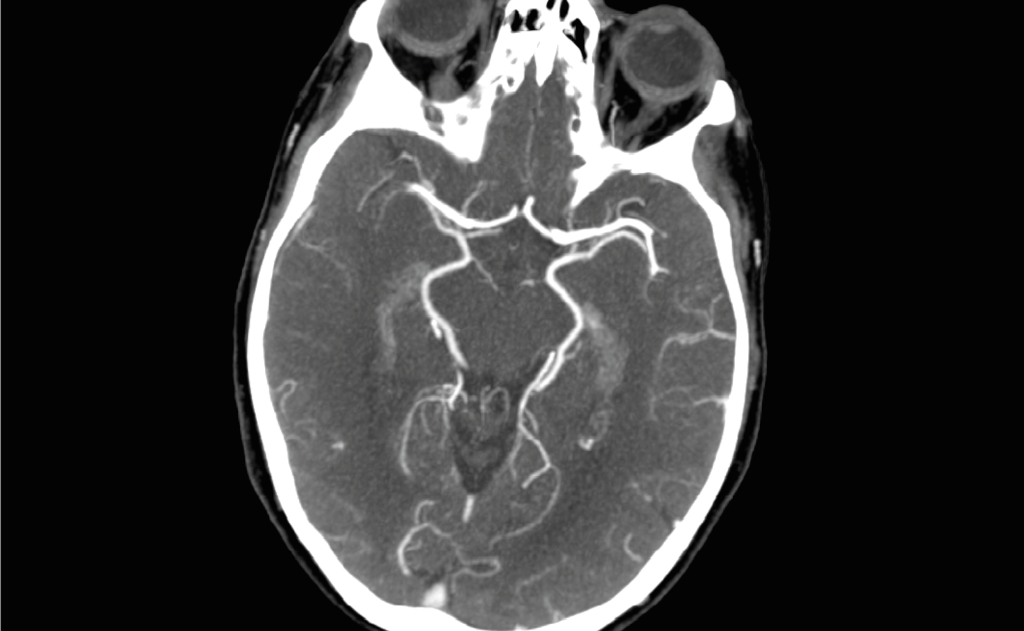
AI triage solution for Pulmonary Embolism and Aortic Dissection receives FDA clearance and CE Mark
Avicenna.AI receives CE Mark for stroke severity assessment AI tool
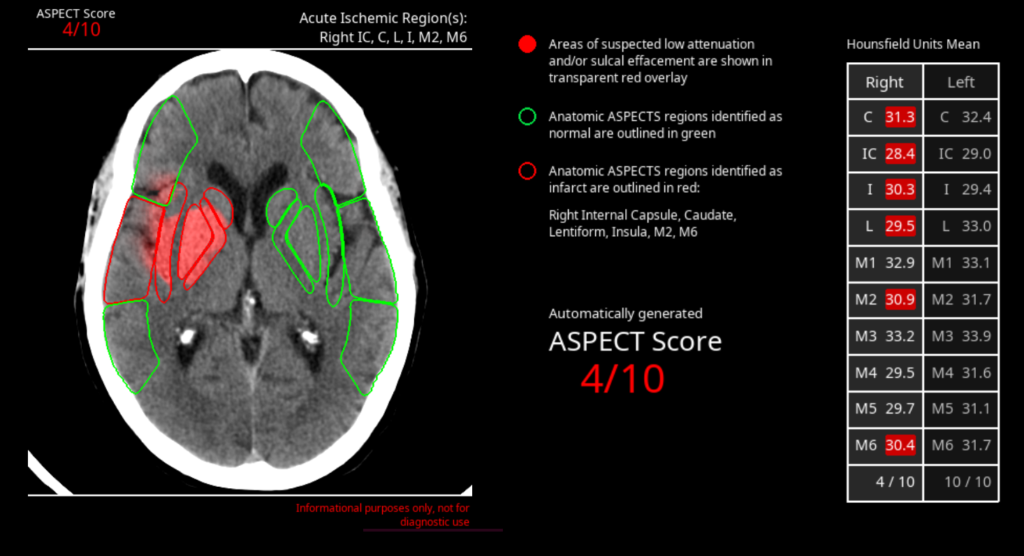
Avicenna.AI receives CE Mark for Automatic ASPECT Scoring AI tool
Arterys & Avicenna.AI Join Forces on AI Stroke Detection

Arterys partners with Avicenna.AI on AI Stroke Detection
